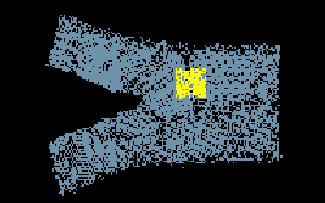
 A nanocystalline
structure of Nickel was examined for this assignment. The lattice
pictured to the left contains a crack, grains, grain boundaries, and vacancies.
The lattice pictured is approximately two layers of atoms thick.
The section in yellow was studied more closely for the specific lattice
characteristics.
A nanocystalline
structure of Nickel was examined for this assignment. The lattice
pictured to the left contains a crack, grains, grain boundaries, and vacancies.
The lattice pictured is approximately two layers of atoms thick.
The section in yellow was studied more closely for the specific lattice
characteristics. Nickel is an element that is usually alloyed with other elements to make it a useful engineering material. Nickel is used for its corrosion resistance and for high temperature resistance, taking advantage of their high melting points and high strengths. Nickel has the crystal structure FCC which can be represented by 4 sub lattices. Pure nickel can be used in corrosion resistance and is typically annealed or cold worked to strengthen. Nickel alloyed with copper is useful in valves, pumps, heat exchangers, shafts, springs, and impellers depending on percentage of Cu and processing method.
A large area of nickel research is in the area of superalloys. A superalloy contains large amounts of alloying elements intended to produce a combination of high strength at elevated temperatures, and resistances to creep. Superalloy nickels are used in jet engines, heat treatment equipment, and gas turbines because of their high temperature strength, and creep resistance.
The symmetry of the nanocrystalline film can be seen on opposite sides of the crack. Also a symmetry is apparent normal to the crack plane. Contained in the picture above are grains, grain boundaries, dislocations, and vaccancies. Grains are the crystalline regions of a solid. Randomly oriented grains exhibit isotropic material properties. The grain boundaries in between the grains act as strengthening mechanisms for the metal. As dislocations move through the lattice under load, the grain boundaries will restrict their movement increasing the strength of the material. Vacancies can be seen and are usually a function of temperature.
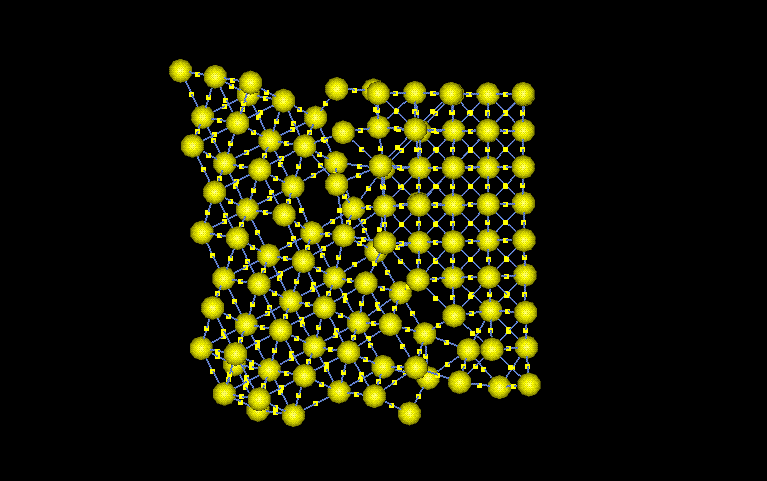 The selected
section was chosen because of its variety of lattice features. The
angle between the grains is around 110°. This large difference
in crystals causes a grain boundary to form between the grains.
Furthermore, as the left side atoms get closer to the boundary, vaccancies
start appearing. There is also a possibility that the distortion
is a dislocation. The dislocation could have been caused by the stress
concentrations around the crack tip. The equilibrium atomic spacing
is 3.52Angstroms. The stress form the crack would cause changes in
this equilibrium spacing. All the features discussed help give Nickel
the mechanical and chemicla properties it will exhibit.
The selected
section was chosen because of its variety of lattice features. The
angle between the grains is around 110°. This large difference
in crystals causes a grain boundary to form between the grains.
Furthermore, as the left side atoms get closer to the boundary, vaccancies
start appearing. There is also a possibility that the distortion
is a dislocation. The dislocation could have been caused by the stress
concentrations around the crack tip. The equilibrium atomic spacing
is 3.52Angstroms. The stress form the crack would cause changes in
this equilibrium spacing. All the features discussed help give Nickel
the mechanical and chemicla properties it will exhibit.